Geology in caves
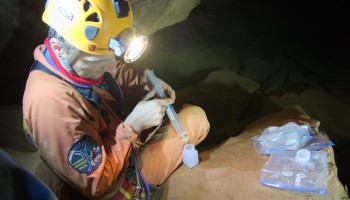
The main objective of these measurements is to determine the chemical composition and physical parameters of cave water in branches of the Sa Grutta caves. Water chemistry is an important indicator of environmental quality and in caves it determines dissolution kinetics and speleothem precipitation. From a speleological point of view it is a useful tool to recognise water coming from recharge areas in the karst aquifer, giving clues as to where to explore next. Physical feature-analysis of cave water is performed in situ at different locations with a Hanna instrument equipped with a probe that measures pH, Electric Conductivity (EC), Total Dissolved Solids (TDS) and temperature. The pH value gives an idea of water acidity and mostly depends on organic compounds and dissolved CO2. Electric Conductivity is a measure of dissolved salts in water, while Total Dissolved Solids represents the water chemical elements content. Water temperature mainly depends on the altitude where the infiltrated rain is collected. Water samples are collected daily in two different bottles, one for cations (positively charged ions) and one for anions (negatively charged ions) from pools of water. Water is
partially analysed (titration with hydrochloric acid) in the evening at camp site to avoid carbonate precipitation. Titration is a measurement of water alkalinity, while for the analysis of anions the sample must be stabilised by acidification with nitric acid.
Operational scenario
Water measurement and sample collection is performed in three pre-defined places and in two new locations chosen during exploration (as indicated on the timeline). A
Hanna instrument is used to measure physical parameters (see procedures).
Water is sampled in two different bottles: one should be left unfiltered and the other filtered using a syringe and a 45 micron filter. Samples are partially treated during evenings at the camp site (the procedure must be carefully executed).
Things to keep in mind
Handling of Water Chemistry tools
The Hanna measurement unit and the upper part of the probe are not waterproof so instruments must be handled with care. The plastic lid that protects the probe can be lost and so should be kept in sight. Sensors must be kept wet at all times so add some water inside the lid before it is placed back onto the probe.
Acidification of water samples for chemical analysis in the laboratory is done with concentrated acid that can burn skin. If burned, wash with large quantities of water.
Water chemistry behaviour
In karst areas, water pH is usually buffered by carbonate to a neutral value of 7. The Electric Conductivity values increase as the water stays in the karst aquifer. The Total Dissolved Solids tells you something about the water-rock
interaction during water flow; water temperature usually increases from the recharge area of the karst system to the spring, and it represents the average annual value of surface temperature. Elemental chemistry discriminates different water sources and allows the reconstruction of the underground river network from where it sunk underground to outlets giving an indication of exploration towards unknown cave passages.
Prof. Jo De Waele, University of Bologna, CAVES science coordinator
Dr. Laura Sanna, Institute for Biometeorology, Sassari, scientist responsible for the CAVES geology experiments



Discussion: one comment
Do you want know why the caves and canyons exist, access: http://www.VenusTerra.com.br/aformacaodoscanions.html and https://www.Venusterra.com.br/ascavernas.html