This story is mirrored from the ESA Web Portal
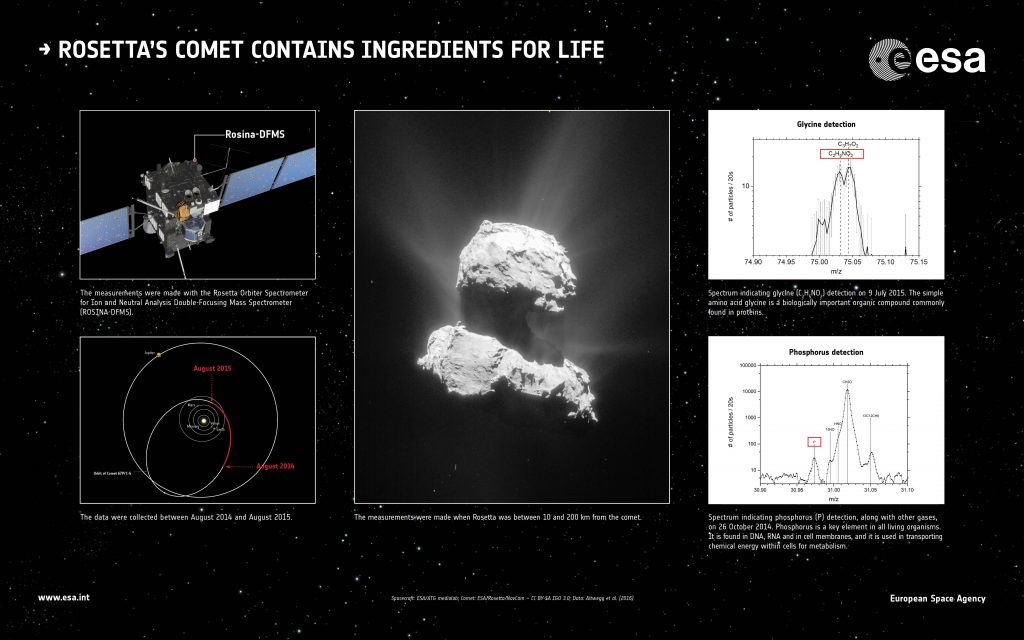
Ingredients regarded as crucial for the origin of life on Earth have been discovered at the comet that ESA’s Rosetta spacecraft has been probing for almost two years.
They include the amino acid glycine, which is commonly found in proteins, and phosphorus, a key component of DNA and cell membranes.
Scientists have long debated the important possibility that water and organic molecules were brought by asteroids and comets to the young Earth after it cooled following its formation, providing some of the key building blocks for the emergence of life.
While some comets and asteroids are already known to have water with a composition like that of Earth’s oceans, Rosetta found a significant difference at its comet – fuelling the debate on their role in the origin of Earth’s water.
But new results reveal that comets nevertheless had the potential to deliver ingredients critical to establish life as we know it.
Amino acids are biologically important organic compounds containing carbon, oxygen, hydrogen and nitrogen, and form the basis of proteins.
Hints of the simplest amino acid, glycine, were found in samples returned to Earth in 2006 from Comet Wild-2 by NASA’s Stardust mission. However, possible terrestrial contamination of the dust samples made the analysis extremely difficult.
Now, Rosetta has made direct, repeated detections of glycine in the fuzzy atmosphere or ‘coma’ of its comet.
“This is the first unambiguous detection of glycine at a comet,” says Kathrin Altwegg, principal investigator of the ROSINA instrument that made the measurements, and lead author of the paper published in Science Advances today.
“At the same time, we also detected certain other organic molecules that can be precursors to glycine, hinting at the possible ways in which it may have formed.”
 The measurements were made before the comet reached its closest point to the Sun – perihelion – in August 2015 in its 6.5 year orbit.
The measurements were made before the comet reached its closest point to the Sun – perihelion – in August 2015 in its 6.5 year orbit.
The first detection was made in October 2014 while Rosetta was just 10 km from the comet. The next occasion was during a flyby in March 2015, when it was 30–15 km from the nucleus.
Glycine was also seen on other occasions associated with outbursts from the comet in the month leading up to perihelion, when Rosetta was more than 200 km from the nucleus but surrounded by a lot of dust.
“We see a strong link between glycine and dust, suggesting that it is probably released perhaps with other volatiles from the icy mantles of the dust grains once they have warmed up in the coma,” says Kathrin.
Glycine turns into gas only when it reaches temperatures just below 150°C, meaning that usually little is released from the comet’s surface or subsurface because of the low temperatures. This accounts for the fact that Rosetta does not always detect it.
“Glycine is the only amino acid that is known to be able to form without liquid water, and the fact we see it with the precursor molecules and dust suggests it is formed within interstellar icy dust grains or by the ultraviolet irradiation of ice, before becoming bound up and conserved in the comet for billions of years,” adds Kathrin.
Another exciting detection made by Rosetta and described in the paper is of phosphorus, a key element in all known living organisms. For example, it is found in the structural framework of DNA and in cell membranes, and it is used in transporting chemical energy within cells for metabolism.
“There is still a lot of uncertainty regarding the chemistry on early Earth and there is of course a huge evolutionary gap to fill between the delivery of these ingredients via cometary impacts and life taking hold,” says co-author Hervé Cottin.
“But the important point is that comets have not really changed in 4.5 billion years: they grant us direct access to some of the ingredients that likely ended up in the prebiotic soup that eventually resulted in the origin of life on Earth.”
“The multitude of organic molecules already identified by Rosetta, now joined by the exciting confirmation of fundamental ingredients like glycine and phosphorous, confirms our idea that comets have the potential to deliver key molecules for prebiotic chemistry,” says Matt Taylor, ESA’s Rosetta project scientist.
“Demonstrating that comets are reservoirs of primitive material in the Solar System and vessels that could have transported these vital ingredients to Earth, is one of the key goals of the Rosetta mission, and we are delighted with this result.”
“Prebiotic chemicals – amino acid and phosphorus – in the coma of comet 67P/Churyumov–Gerasimenko”, by K. Altwegg et al is published in the journal Science Advances.







Discussion: 22 comments
As if biochemistry had been always out there… Not evolutive, not a subproduct of life, but a building block.
No words at the moment. Just AWE!
Congratulating all the ROSINA Team, specially Kathrin, Hervé et al.
Fully open the document 🙂 Will read…
50 new doubts and questions for every new answer.
The inference of glycine is an important milestone.
Interesting would be additional hints to a presence of nucleotide bases, as another important ingredient for life, or to porphyrine building blocks.
Are all dectected organic molecules at 67P still aliphatic?
Hi Gerald,
I think all 67P positively identified organics are aliphatic, but many longer chain organics have been detected and, I think, just take longer to confirm as aromatic or otherwise, although Deep Impact released many PAH’s. Among other things.
Hi Marco,
thanks, that’s my latest state, too. But I thought/hoped, that I might have overlooked some update.
It is interesting to speculate on how much chemistry might take place during the time a comet is passing through the Earth’s atmosphere. These basic building blocks are going to be in an aqueous environment at “room temperature” and above in certain parts of the comet for small amounts of time. Add to that the “catalytic” effect of tiny interplanetary dust grains, the comet is going to be a fast moving chemical synthesis lab. There is the opportunity for highly complex molecules to form in this brief period of high energy and high pressure.
How much survives to reach the ground is another story, as is what happens if the comet lands in water. Relative abundances and types of complex organics generated by comets as they disperse into the primitive Earth environment are going to be considerably different to those of a comet in space.
I have a question about the connection of these findings to those regarding clathrates. Is it true that only small compounds can be found in clathrate form, and hence that larger compounds like these amino acids must be in some kind of unbound form? In which case, could they have formed while the comet was going around the Sun?
I am also curious about other complex organic compounds. For example, polyoxymethylene was mentioned from Philae Ptolemy, and Ian Wright had some more exotic names. Is there confirmation for these compounds? Are there mass spectra which, so far, we have failed to identify because the compound may not be one which is easily found on Earth?
Regarding the mass spectra: The spectra I’ve seen (scattered in previous blog posts and elsewhere) contain almost any fragments of a parent molecule you can think of.
There are at least two approaches how to interprete those:
– Either reassemble the parent compound from the fragments, or
– Use reference data, e.g. from NIST, to do some Rietveld refinement, or similar techniques to find the initial composition.
The second approach certainly suffers from the possible lack of reference data, but is likely to provide accurate results, if appropriate reference data have been used. Otherwise you’re likely to get large residual errors.
I guess, that an experienced scientist in the respective field “sees” good candidate components, when looking at a spectrum. I can at least do so without much experience in these spectra, and I see compounds I’ve never heard of before. So I’m sure, experts can do much better.
If there is an unknown compound, you can do theoretical calculations to obtain first estimates, how such a compound should decompose into fragments; and so you get a first check of your idea.
The masses are only up to about 100 atomic units. So there is only a finite number of possible molecule fragments which can be detected. The mix of fragements resolves (most) ambiguities caused by fragements of equal atomic weights.
Regarding the clathrates: Clathrates, as I understand them, are ices which contain single molecules of a different species enclosed in cages, which still obey a crystal lattice structure.
Those single molecules should have been a gas, or in solution, when enclosed into the ice.
Substances consisting of large molecules are usually solid at a temperature low enough for the solvent to freeze to ice. Then it’s difficult to obtain individual molecules to be enclosed into a cage.
And large molecules would also destroy the lattice structure of the ice.
We would then eventually get an ice – dust mix, instead of a chemkcal compound, or kind of a frozen emulsion, better called a solid colloid or a solid sol:
https://en.wikipedia.org/wiki/Colloid#Classification
Alien molecules, Kamal 🙂
https://en.wikipedia.org/wiki/Van_der_Waals_molecule
100 is a NATURAL number 😉
Biochemistry is plenty of those weakly bound constructs and foldings.
Minute environmental changes transmuting them into different Beasts.
In that sense Van der Waals chemistry perform as a ‘transmission medium’.
…..
“…contain almost any fragments of a parent molecule you can think of…”
Gerald’ comment incommensurately enrich the plausible formative scenarios.
“…and hence that larger compounds like these amino acids must be in some kind of unbound form?”
Intriguing question. If uncaged Then No Time Capsule arguments.
As for me, thinking of Proto-Planetary Disks as conformed of already ‘predigested’ material kind of solves the issue:
Material from several failed core generations. Slightly cooked at more than one epoch.
This is my pretty speculation, of course 😉
Got the former ‘environmental’ idea from:
https://en.wikipedia.org/wiki/Marine_snow
And several recent papers abstracts about molecular clouds.
“Marine snow aggregates are porous, however, and some particles are able to pass through them.” 😉
“Differential Settling
This form of aggregation involves particles sinking at different rates and their collision to form aggregates”
New papers use to talk about ‘failed’ cores. How does it look a failed core?
Ah! haha 😉
[Maybe a little ‘brownish’?]
Next point is just ‘food for thought’ in Carl Sagan’ Sense:
Question is not If Aliens exist or not, but if Life is Alien per se.
“The discovery of electric bacteria shows that some very basic forms of life can do away with sugary middlemen and handle the energy in its purest form – electrons, harvested from the surface of minerals. ‘It is truly foreign, you know,’ says [USC Kenneth] Nealson. ‘In a sense, alien.'”
https://www.newscientist.com/article/dn25894-meet-the-electric-life-forms-that-live-on-pure-energy/
Now, trying to clear my mental…
The gas an particulate directly directly below the ‘shocked’ bow has a little resemblance to an ‘atmospheric behavior’.
And that conic ‘atmosphere’ could also be related to ‘necking’ phenomena.
Some months ago played with the idea of Sun ‘sipping’ 67P from a peasant Brown..
Now Lund Observatory, Sweden
Laboratoire d’Astrophysique de Bordeaux, France
& Laboratoire d’Astrophysique de Bordeaux, France
Iterating with refreshing concepts on planet ‘sipping’:
Abstract Resource: https://mnrasl.oxfordjournals.org/content/460/1/L109
Congratulations to Alexander J. Mustill, Sean N. Raymond, Melvyn B. Davies and collaborators!
And very well journaled by Cecilia Schubert via: https://phys.org/news/2016-05-theft-planet-solar.html
“First. You need a certain star type, at a certain age.
Second. You need a certain planet, at a certain distance of star…”
What if We omit the Second pass?
Could biochemistry develop at certain layers of very cold Brown Stars? And then that biochemistry just ‘snow’ down to deeper layers?
” The First Spectrum of the Coldest Brown Dwarf”
Andrew Skemer, Caroline Morley, Katelyn Allers, et al.
https://arxiv.org/abs/1605.04902
via https://phys.org/news/2016-07-astronomers-evidence-clouds-spectrum-coldest.html