This news release is mirrored from the ESA portal, published on the occasion of the publication of the paper “Molecular nitrogen in comet 67P/Churyumov-Gerasimenko indicates a low formation temperature,” by M. Rubin et al. in the journal Science.
ESA’s Rosetta spacecraft has made the first measurement of molecular nitrogen at a comet, providing clues about the temperature environment in which Comet 67P/Churyumov–Gerasimenko formed.
Rosetta arrived last August, and has since been collecting extensive data on the comet and its environment with its suite of 11 science instruments.
The in situ detection of molecular nitrogen has long been sought at a comet. Nitrogen had only previously been detected bound up in other compounds, including hydrogen cyanide and ammonia, for example.
Its detection is particularly important since molecular nitrogen is thought to have been the most common type of nitrogen available when the Solar System was forming. In the colder outer regions, it likely provided the main source of nitrogen that was incorporated into the gas planets. It also dominates the dense atmosphere of Saturn’s moon, Titan, and is present in the atmospheres and surface ices on Pluto and Neptune’s moon Triton.
It is in these cold outer reaches of our Solar System in which the family of comets that includes Rosetta’s comet is believed to have formed.
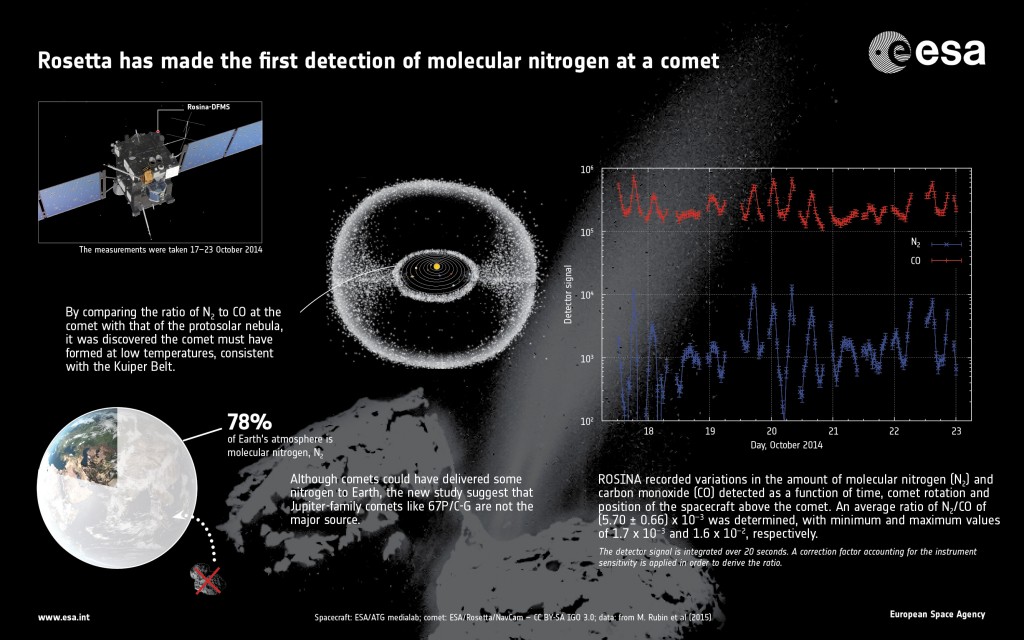
The new results are based on 138 measurements collected by the Rosetta Orbiter Spectrometer for Ion and Neutral Analysis instrument, ROSINA, during 17–23 October 2014, when Rosetta was about 10 km from the centre of the comet.
“Identifying molecular nitrogen places important constraints on the conditions in which the comet formed, because it requires very low temperatures to become trapped in ice,” says Martin Rubin of the University of Bern, lead author of the paper presenting the results published today in the journal Science.
The trapping of molecular nitrogen in ice in the protosolar nebula is thought to take place at temperatures similar to those required to trap carbon monoxide. So in order to put constraints on comet formation models, the scientists compared the ratio of molecular nitrogen to carbon monoxide measured at the comet to that of the protosolar nebula, as calculated from the measured nitrogen to carbon ratio in Jupiter and the solar wind.
That ratio for Comet 67P/Churyumov–Gerasimenko turns out to be about 25 times less than that of the expected protosolar value. The scientists think that this depletion may be a consequence of the ice forming at very low temperatures in the protosolar nebula.
One scenario involves temperatures of between roughly –250ºC and perhaps –220ºC, with relatively inefficient trapping of molecular nitrogen in either amorphous water ice or cage-like water ice known as a clathrate, in both cases yielding a low ratio directly.
Alternatively, the molecular nitrogen could have been trapped more efficiently at even lower temperatures of around –253ºC in the same region as Pluto and Triton, resulting in relatively nitrogen-rich ices as seen on them.
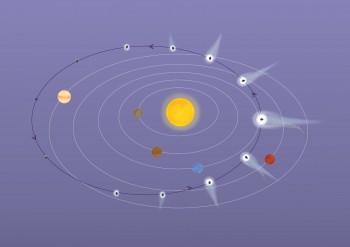
Comet 67P/Churyumov-Gerasimenko is a Jupiter-family comet. Its 6.5 year journey around the Sun takes it from just beyond the orbit of Jupiter at its most distant, to between the orbits of Earth and Mars at its closest. The comet hails from the Kuiper Belt, but gravitational perturbations knocked it towards the Sun where interactions with Jupiter’s gravity set it on its present-day orbit. Credits: ESA
Subsequent heating of the comet through the decay of radioactive nuclides, or as Rosetta’s comet moved into orbits closer to the Sun, could have been sufficient to trigger outgassing of the nitrogen and thus a reduction of the ratio over time.
“This very low-temperature process is similar to how we think Pluto and Triton have developed their nitrogen-rich ice and is consistent with the comet originating from the Kuiper Belt,” says Martin.
The only other body in the Solar System with a nitrogen-dominated atmosphere is Earth. The current best guess at its origin is via plate tectonics, with volcanoes releasing nitrogen locked in silicate rocks in the mantle.
However, the question remains as to the role played by comets in delivering this important ingredient.
“Just as we wanted to learn more about the role of comets in bringing water to Earth , we would also like to place constraints on the delivery of other ingredients, especially those that are needed for the building blocks of life, like nitrogen,” says Kathrin Altwegg, also at the University of Bern, and principal investigator for ROSINA.
To assess the possible contribution of comets like Rosetta’s to the nitrogen in Earth’s atmosphere, the scientists assumed that the isotopic ratio of 14N to 15N in the comet is the same as that measured for Jupiter and solar wind, which reflects the composition of the protosolar nebula.
However, this isotopic ratio is much higher than measured for other nitrogen-bearing species present in comets, such as hydrogen cyanide and ammonia.
Earth’s 14N/15N ratio lies roughly between these two values, and thus if there was an equal mix of the molecular form on the one hand, and in hydrogen cyanide and ammonia on the other in comets, it would be at least conceivable that Earth’s nitrogen could have come from comets.
“However, the amount of nitrogen found in 67P/Churyumov–Gerasimenko is not an equal mix between molecular nitrogen and the other nitrogen-bearing molecules. Rather, there is 15 times too little molecular nitrogen, and therefore Earth’s 14N/15N ratio cannot be reproduced through delivery of Jupiter family comets like Rosetta’s,” says Martin.
“It’s another piece of the puzzle in terms of the role of Jupiter-family comets in the evolution of the Solar System, but the puzzle is by no means finished yet,” says ESA’s Rosetta project scientist, Matt Taylor.
“Rosetta is about five months away from perihelion now, and we’ll be watching how the composition of the gases changes over this period, and trying to decipher what that tells us about the past life of this comet.”
Notes
“Molecular nitrogen in comet 67P/Churyumov-Gerasimenko indicates a low formation temperature,” by M. Rubin et al is published in the 20 March issue of the journal Science. 10.1126/science.aaa6100
ROSINA is the Rosetta Orbiter Spectrometer for Ion and Neutral Analysis instrument and comprises two mass spectrometers: the Double Focusing Mass Spectrometer (DFMS) and the Reflectron Time of Flight mass spectrometer (RTOF) – and the COmetary Pressure Sensor (COPS). The measurements reported here were conducted with DFMS. The ROSINA team is led by Kathrin Altwegg of the University of Bern, Switzerland.
An average ratio of N2/CO = (5.70 +/-0.66) x 10–3 was determined for the period 17–23 October 2014. The minimum and maximum values measured were 1.7 x 10–3 and 1.6 x 10–2, respectively. Because the amount and composition of the gases change with comet rotation and position of the spacecraft with respect to the comet’s surface, an average value is used.
The 14N/15N ratio for the N2 in Comet 67P/Churyumov–Gerasimenko is assumed to be 441, the value for the protosolar nebula as measured from Jupiter and the solar wind, while the corresponding value for nitrogen in hydrogen cyanide and ammonia is 130, as measured at other comets. The value for the Earth’s nitrogen is 272.







Discussion: 44 comments
One would have to assume that given the volatility of Nitrogen gas, that 67P’s previous close approaches to the Sun have led to the majority of the Nitrogen in the near surface layers to have already sublimated away. If the theory of deeper ice phase changes proves viable , considerable amounts of Nitrogen and Carbon Monoxide have been lost from much deeper in the comet also.
These two “super volatiles” are most likely largely responsible for major activity, that is more vigorous than we currently see, so if they have already been depleted by earlier orbits’ intense activity, this could explain the lower than anticipated activity Matt Taylor’s blog mentioned. Until further mass loss enables heat energy to reach any different remaining sources of these two compounds, that may continue to be the case.
Wow great, thanks! Just today a few hours ago I’ve been pondering about exactly this topic.
The 14/15 ratio is quoted as ‘assumed’.
Is ROSINA able to measure it?
The problem may be an inability to resolve N15 from
N14-H and 12CH3 or C13H2- coming ng from organics etc.
Anyone know?
(In HCN and NH3 it may have been measure via the IR rather than the mass spectrum, which you can’t do for N2; again, anyone know?)
They should be able to measure the mass defect of the atomic nuclei, since
m / Delta m > 3000,
according to this paper:
https://link.springer.com/article/10.1007/s11214-006-8335-3#page-1
Thanks, thats the number I was missing.
3000 is certainly good enough to resolve them, with atomic mass/atomic number = 1.00782505 for 1H, exactly unity for 12C of course, and 1.0002195718 for 14N.
So CH3 looks well resolved.
But its only a bit over twice the ‘resolution’ for NH, line overlap might be an issue if the NH cracking fraction is strong.
But it looks measureable as you say; so I wonder why ‘assumed’; maybe not enough counts on the rare isotope peak?
“The 14N/15N ratio for the N2 in Comet 67P/Churyumov–Gerasimenko is assumed to be 441 …
… the corresponding value for nitrogen in hydrogen cyanide and ammonia is 130”.
Hydrogen cyanide and ammonia probably decompose during the measurement, such that you get N peaks from N2, CN, and NH3. Due to the very different 14N/15N ratios in N2 and NH3, and the much higher abundance of CN and NH3 than of N2, decomposing the N+ peak in different source molecules is probably tricky:
You probably need to look accurately at N2+,N+, C+, CN+, NH3+,NH2+,NH+,H+,H2+, maybe more species, and their isotopes,…
Just my guess from a distance without knowing the actual raw data.
Sorry, saw K.Altweggs post just after posting.
Gerald. Most mass specs use a standard ionisation energy (70eV from distant memory) and one has a ‘cracking pattern’ for the species. They are on the web. I’ve used low resolution quadrupole mass specs a lot for vacuum system residual gas analysis, but never high res.
For ammonia
17/100%, 16/80, 15/8 and 14/2
And for nitrogen
28/100% and 14/7
I think the meaning is pretty clear?
So if there is much ammonia about that 15/8% NH peak is going to be strong and close to the 15N.
But another strategy would be to look at 15N14N at 29, I don’t think much interferes there, except some relatively heavy organics?
In practice of course you will fit all the species and their ground-calibrated cracking patterns in one hit, not just ‘look at 29’ or whatever. But the statistics could well become a limiting factor for rare isotope peaks you are trying to unscramble from overlaps.
We’ll have to await the papers 🙁
Thanks, Harvey for the crack patterns! Yes, I understand, molecular mass / relative peak area (abundance).
I agree, to get the best fit, one will try to minimize square error sums for the whole spectrogram, or at least up to some upper mass limit, including appropriate deconvolution, considering FWHM calibration, background etc.
ROSINA is able to separate 15N from NH. However, densities at the moment do not allow to see 15N. We have to wait for being closer /having more outgassing.
Many thanks, sorry I saw this after my other post. You D guessed it was probably shortage of signal.
Eclipse: sun, earth, moon and 67p with little comatose boy Philae – all more or less aligned right now.
I thought the presence of the more abundant CO told pretty much the same story about cold formation?
Must say that so many of the articles posted have been fantastic, and all articles much appreciated, and it’s great to know what instruments are collecting what data and to get updates on that. But sorry, the conclusions being drawn in this article seem to be based on so many qualifiers, assumptions, and ifs-thens as to make them virtually meaningless. For instance (all caps are my highlights, parenthesis my comments):
“Its detection is particularly important since molecular nitrogen is THOUGHT to have been the most common type of nitrogen available when the Solar System was forming. In the colder outer regions, it LIKELY provided the main source of nitrogen that was incorporated into the gas planets.
It is in these cold outer reaches of our Solar System in which the family of comets that includes Rosetta’s comet is BELIEVED to have formed.
“Identifying molecular nitrogen places important constraints on the conditions in which the comet formed, because it requires very low temperatures to become trapped in ICE,” (so this statement and the many that follow only applies if there is ice on the comet, which unless I’m mistaken has not been proven yet?)
The trapping of molecular nitrogen in ice in the protosolar nebula is THOUGHT to take place at temperatures similar to those required to trap carbon monoxide.
The scientists THINK that this depletion MAY be a consequence of the ice forming at very low temperatures in the protosolar nebula.
ONE SCENERIO involves temperatures of between…
Alternatively, the molecular nitrogen COULD have been trapped…
Subsequent heating of the comet through the decay of radioactive nuclides, or as Rosetta’s comet moved into orbits closer to the Sun, COULD have been sufficient to trigger outgassing…
The title of the article is a nice clear statement based on scientific data. As for pretty much the rest of the article, the phrase “Building castles in the sky” comes to mind.
Empirical science is about statistics, inference, models, deductive methods, interpretation, it’s not exclusively an axiomatic theory with strictly logical inductive rules.
So during ongoing exploration, and during data collection and during ongoing calibration and data reduction, you can just release preliminary results.
It can take years to get evident or even definitive results.
But for everyone except maybe the respective instrument scientists, those intermediate information is better than waiting years just to go from a 2.8 sigma to a 3.1 sigma, or from a 4.6 sigma to a 5.3 sigma confidence, without changing much the essential information.
So thanks to the teams for releasing intermediate data and interpretations! No need to get everything absolutely waterproof at the current state of ongoing exploration.
Now Gerald, it’s a little disingenuous to try to frame my post as quibbling about the article not presenting the exact measurement for sigma. To it’s credit, this article does not state suppositions as declared facts, and is more a journey into speculations about some possible perhaps maybes. But I think it highlights the fact that much of cosmology is based on assumptions who’s conclusions are often mistaken for (or presented as) fact. And it’s devilishly difficult to separate one’s assumptions from what one holds as facts, but the wording of the article makes it clear that ALL the things I highlighted in my post above are assumptions.
Not to claim options as facts is a good practice in planetology.
Well said.
Hi Emily. Thanks to you, ESA and ROSINA Team about this so much expected data. At first tough, that graph suggest about CO being kind of ‘blended’ into the ice, and about N2 being more related to surface.
Maybe nitrogen is -someway- significantly more covalently attached to dust than CO.
Speculating N2 graph is going to approach CO graph at perihelion. [Higher exposed surface, including dust-o-sphere].
Have M Rubin et al or anyone else ( ie Matt Taylor) considered any other possible origin for molecular nitrogen and explanation for the nitrogen/ nitrogen ratio. It seems that everybody has simply assumed that the primordial origin hypothesis is correct and carried on from there
In fact molecular nitrogen is a product of combustion of ammonia in oxygen, which also produces water and heat. And two natural sources of N15 are the positron emission of oxygen15 and the beta decay of carbon15. Neutron capture may also occur in high energy environments.
And let us not overlook the fact that typically N14 makes up in excess of 99.6 % of natural nitrogen so tiny changes in the quantity of N15 are all that is occurring whether or not the ratio is described as much higher or much lower. Not an open and shut case by far.
I could probably list twenty ways to generate molecular nitrogen if you give me a couple of minutes. Yes, if you burn NH3 in O2 you will get water & N2. What possible relevance has that? There is no sign of high concentration of molecular O2, & no conditions under which it would be likely to ‘burn’.
Im very well aware its a minor constituent, I’ve bought enough 15N compounds over the years at considerable cost!
At the oment they are *assuming* a given isotope ratio, & it appears (thanks to Gerald’s post) that they can resolve 15N from CH3 & probably NH, if they have a good enough count rate on the peak.
Nothing is ‘open on shut’ on this; we actuually have no data; but I very much doubt 2NH3+3O2->N2+3H2O has anything to do with it.
Its possible 15N could also be measured from the IR spectrum (of NH3, HCN for example, but not N2) if the S/N & resolution are good enough. I’d have to dig around in the literature to see if its been done for a comet (easy in the lab.)
The obvious relevance that seems to escape you Harvey is that ammonia is a ubiquitous constituent of the mix of chemicals that coat the surface of comet nuclei. It would be high up in the list of possible sources of molecular nitrogen, should an oxygen combustion reaction occur, way above an imagined primordial origin in the hypothetical solar nebula.
The potential source of oxygen, detected yet or not and doubted by you or otherwise, is the energetic impact of the persistent current of solar protons breaking Si-O bonds in the rock of the nucleus.
Reality takes no account of preferences even if they are those of the consensus peer group. Evidence is what counts and in due course the evidence will overwhelmingly speak for itself.
Retry.
‘Combustion’ is primarily a high density phenomenum because it requires collisions.
Solar proton sputtering produces *minute* concentrations of atomic oxygen, due to its low density and low sputter efficiency.
So the conditions to support ‘combustion’ simply don’t exist.
Some of the N2 might I guess originate from NH3, though I’m sure there would be other sources. Solid state ion induced reaction might even be involved, concentrations are tiny.
But ammonia/oxygen ‘combustion’ makkes no sense whatever.
Indeed, evidence is what matters; Aside from a vague perceived similarity between 67P jets & discharges – a similarity largely in the eyes of people who seem to know little about discharges – There is absolutely NO evidence in favour of the EU theories. Sure, ‘standard’ theories have their problems; but they have the ‘nerve’ to put up models, numbers, which the evidence can challenge. EU never, ever does that. Neither does it ever reply to ‘difficult’ questions; can we have some answers to the issues of, at the most basic level, how this discharge ‘works’? Numerically, charged body discharging is daft; yet we have no ‘circuit’; so how, at that most basic level, does this CW discharge *work*?
I replied to your comments Harvey but it did not see the light of day, I expect because they see us as off topic regarding this post. So getting back specifically to the nitrogen issue your assertions about low density and oxygen production are not backed up by results. They are your opinion. My opinion is that combustion is perfectly viable. I contend that that natural plasma effects occur in the coma which probably increase the proton density by many orders of magnitude. We await the results to confirm or refute that. Until they are forthcoming there is no reason to dismiss the possibility that the detected molecular nitrogen is a combustion product. It is the most likely origin.
You say there is no evidence to support EU “theories”. That is because there is little evidence published as yet. Wait and see. In the meantime I suggest you research the EU explanation a little more deeply because you don’t seem to have grasped it at all. The current you seek is the flow of charged particles known as the solar wind, protons from the Sun, electrons to the Sun and a persistent radial electric field throughout the heliosphere. The comet with its varying charge induced by its elliptical orbit is moving within that field.
It is likely that nitrogen production will continue approaching perihelion. Qualitative results on it and other combustion products will indicate whether combustion is complete or incomplete and that in itself will be a good indicator of availability of oxygen. Look out for CO, HCHO and atomic H, classic incomplete products. Either way the water production volume will be easily accounted for.
O15 decay may be a releveant source of N15 in the context of nuclear fusion within stars, see Bethe-Weizsäcker cycles / CNO cycles suggested for some kinds of stars:
https://en.wikipedia.org/wiki/CNO_cycle
I don’t see a natural source of C15, but can’t rule out, that there might exist some.
But consider, that O15 has a half-life of 122 seconds, and C15 a half-life of 2.449 seconds.
https://en.wikipedia.org/wiki/Isotopes_of_oxygen
https://en.wikipedia.org/wiki/Isotopes_of_carbon
So (almost) any C15 or O15 decayed long before the formation of the comet; the resulting N15 would therefore be considered as prestine.
There will form some radio-isotopes due to hits by high-energy cosmic rays.
But they should leave typical “isotopic fingerprints” to be discernible from prestine N15.
Most cosmic rays can’t penetrate the comet more than several meters. So the outer layer should contain most of these altered isotopes; the same layers that sublimate first. Hence most of those isotopes are probably already gone. I guess, that this latter reason will have led to the neglect of the cosmic rays alteration products.
Free neutrons are rare and short-lived (half-life less than 15 minutes).
https://en.wikipedia.org/wiki/Neutron#Free_neutron_decay
They form naturally in spontaneous fission of heavy elements like uranium, which usually is rare in nature, even shortly after a supernova explosion, although it’s more abundant near those events.
Combustion of ammonia in an oxygen environment doesn’t look likely in the history between supernovae and formation of the comet.
The scientist are aware of these possible origins of N15, but besides maybe the CR origin it’s not too relevant, since the N14/N15 ratio of other objects in our solar system is presumed to be of similar origin.
Other factors influencing the N14/N15 ratio may be fractional sublimation or fractionation by diffusion.
The vapour pressure curves of N2 and CO are really quite close to each other. However their binding properties on dust grains etc might differ much more.
It might be of interest to know that the most commonly used stable isotopes,(aside from deuterium) 13C 15N and 18O are produced by multiple cryogenic distillation of a suitable species like CO etc. Not sure what they use for N, possibly NH3 or N2 itself. The apparatus is a long column which feeds lower boiling point fractions one way and higher the other with many stages. (A bit like a tiny version of some oil refinery plant, much less throughput but many more stages.) They take a long time to equilibrate, but then produce a steady output, which is why those isotopes, whilst expensive, are not that bad to buy.
Relevant, because one can imagine that many such cycles could conceivably occur in a cometary environment, leading to isotopic fractionation.
Hi Harvey. There is a kind of fluid transit in which the second or third molecular gas layer ‘walks’ down over the first one. No need of liquid state to decant. Of course, this is still far from established science.
So, we thought that comets “seeded” Earth with a lot of elements like H2O or N2 but up to now, the measures done on site show that both H2O and N2 came from something else than a bombing during Earth early days.
There are also water-bearing asteroids; there are several families of comets with varying isotopic composition. So the game is still open.
Maybe [just slightly] Earth also is a former icy -migrated- planet 😉
Maybe planets are formed well before than [planetary?] disks.
Polishing this idea a little more: When a disk is accreted around a forming star, Big planets an moons are already there, in the matter composing the disk. Just proposing one more among lots of plausible scenarios.
Possible:
https://en.wikipedia.org/wiki/Rogue_planet
No future star has a guaranteed future as such. Inside the dust cloud should be a lot of condensates of all sizes gravitationally attracting each other.
Jupiter almost got it. Jupiter-Saturn gravitational play must be a ‘tricky’ one.
Yes, tricky, indeed.
https://en.wikipedia.org/wiki/Planetary_migration
https://en.wikipedia.org/wiki/Orbital_resonance
Thanks a lot for the links, Gerald 🙂
Nothing says about harmonies between Jupiter and Saturn, but you should be right on cause. Some self balancing act around there.
From a Saturn perspective. This is binary system.
2 and 3 layer square ice crystals sandwiched on graphene. Room temp. Not theoretical 🙂
https://www.nature.com/nature/journal/v519/n7544/full/nature14295.html
Thanks for that! Has been unclear, whether this modification is stable under normal pressure.
‘Adsorpted’ should be handled as another state of mater.
………………………….
A cloud of dust having a very similar [non-distributed] temperature should exhibit very peculiar behavior, too.
Any of you have experimented with water heated just before boiling point? The least of disturbances have ‘catastrophic’, extensive, wave-propagating effects.
Heating water above it’s boiling point can cause boiling delay:
https://en.wikipedia.org/wiki/Superheating
…more generally known as metastability:
https://en.wikipedia.org/wiki/Metastability
An interstellar cloud of gas may get instable when cooling down:
https://en.wikipedia.org/wiki/Jeans_instability
Possible, that there may exist metastable states.
Thanks for the links, Gerald. Didn’t know a full discipline exists for the study of those phenomena. After reading them, suppose Metastability an important issue at comet studies.