This entry is based on the Southwest Research Institute’s press release and covers the results of the ROSINA instrument – the Rosetta Orbiter Spectrometer for Ion and Neutral Analysis – that were published today in the journal Science.
Scientists working on the ROSINA instrument have discovered that Comet 67P/Churyumov-Gerasimenko’s atmosphere, or coma, is much less homogenous than expected and that comet outgassing varies significantly over time.
“If we would have just seen a steady increase of gases as we closed in on the comet, there would be no question about heterogeneity of the nucleus,” says Dr. Myrtha Hässig, lead author of the paper and a postdoctoral researcher at Southwest Research Institute in San Antonio. “Instead we saw spikes in water readings, and a few hours later, a spike in carbon dioxide readings. This variation could be a temperature effect or a seasonal effect, or it could point to the possibility of comet migrations in the early Solar System.”
“Our whole concept of the variability of volatile release at comets will change based on this paper, which will have significant impact on our understanding of comet formation and evolution,” says Dr. Hunter Waite, a program director and planetary scientist at SwRI.
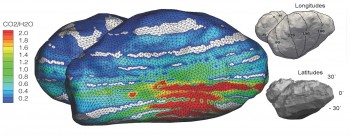
Rosetta scientists measuring the composition of comet 67P’s atmosphere or coma discovered that it varies greatly over time. Large fluctuations in composition in a heterogeneous coma indicate day-night and possibly seasonal variations in the major outgassing species: H2O, CO, and CO2. The red region where CO and CO2 dominate is a part of the comet that is poorly illuminated, indicating a complex coma-nucleus relationship where seasonal variations may be driven by temperature differences just below the comet surface.
Shape model credit: ESA/Rosetta/MPS for OSIRIS Team MPS/UPD/LAM/IAA/SSO/INTA/UPM/DASP/IDA
Comets are small Solar System bodies with a nucleus composed of ice, dust, and small rocky particles. As comets approach the Sun along their orbit, they heat up and outgas, displaying visible atmospheres and tails. Comets contain some of the best-preserved material from the formation of our planetary system, offering clues about physical and chemical conditions that existed in the early Solar System.
Rosetta has been studying Comet 67P/C-G since it began approaching the four kilometre wide world last year, finally arriving at a distance of 100 km on 6 August 2014.
“From a telescope, images of a comet’s atmosphere suggest that the coma is uniform and does not vary over short periods of hours or days. That’s what we were expecting as we approached the comet,” said Dr. Stephen Fuselier, a director in the SwRI Space Science and Engineering Division and the lead U.S. co-investigator for ROSINA’s DFMS instrument. “It was certainly a surprise when we saw time variations from 200 km away. More surprising was that the composition of the coma was also varying by very large amounts. We’re taught that comets are made mostly of water ice. For this comet, the coma sometimes contains much more carbon dioxide than water vapour.”
Indeed, ROSINA data indicate that the H2O signal is strongest overall; however, there are periods when the CO and CO2 rival that of H2O.
“These large fluctuations in composition in a heterogeneous coma indicate diurnal or day-night and possibly seasonal variations in the major outgassing species,” says Hässig. “When I first saw this behavior, I thought something may have been wrong, but after triple-checking the data, we believe 67P has a complex coma-nucleus relationship, with seasonal variations possibly driven by temperature differences just below the comet surface.”
The nucleus of 67P/C-G consists of two lobes of different sizes, connected by a neck region. This complex, “rubber-ducky” shape likely plays a key role in this variation, as different portions of the nucleus face the Sun during the comet’s 12.4 rotation cycle. If the coma composition reflects the composition of the nucleus, variations suggest that the nucleus may have formed by collision of smaller bodies that originated from very different regions of the early Solar System. This discovery could revolutionize theories about comet formation and the relationship between comet composition and the location of its origin.
As the comet continues its 6.5-year journey around the Sun, Rosetta scientists will be able to consider seasonal variations as well.
“Time Variability and Heterogeneity in the Coma of 67P/Churyumov-Gerasimenko” is published in the 23 January issue of the journal Science.
The ROSINA team is led by Kathrin Altwegg of the University of Bern, Switzerland.







Discussion: 6 comments
Three article refs! Good science!
I’ll note that the High:Low CO2/H2O boundary is just South of the Imhotep Plain, at first look.
–Bill
Fascinating. Wonder if the differing ‘components’ can be identified from various parts of the Kuiper Belt?
finally… and I did subscribe to science today to read the article,
for details as is this the detection of neutral water, or inferred, same with the other compounds.
now that the paper is written, how about giving some ‘time lapse’ about the development of the outgassing
would really be interesting to see how this comet starts to activate
I know from working with vacuum, water stays at this .001 forever until all the water is gone, down to liquid nitrogen temps
is there any He coming out (it won’t be liquid in the comet core, but there might be some trapped)?
would love to see something like an IBM DX interactive rendering of the time development of all the different things that come out of this ‘sponge’….
This covers what Katherine said at the AGU last month, but the image in Emily’s post is new. Although it looks like the surface composition, it is actually the gases above the surface in the coma. The MIRO paper needs to be read in conjunction with these results as it talks about the energy flux hitting the comet from the sun and heat reflected from the surface of the comet, as well as giving estimates for the temperature gradients below the surface. The top mm of the surface can vary by as much as 50K during one comet day, but below that layer such is the high insulation value of the dust, the temperature can be 30K lower.
The daily sunlight energy cycle only affects the top few cm of the surface, the seasonal variations affect maybe the top metre or so and the orbital energy flux changes from nearest to farthest point to the sun, may only reach a few metres below the surface. The conclusion being that all the activity we see up until now is from less than 1% of the comet’s surface and maybe only the top few centimetres. This means although OSIRIS and VIRTIS can only verify surface ice in small patches, that and the larger amounts of ices very close to the surface in the Hapi (neck) region, are more than enough to account for all the activity we have seen so far, but the energy appears not just to be coming from sunlight.
Thermal inertia plays a role to delay activity, by the time the energy from the sun gets down to where the ices are, a few mm below the surface, the area may well be in shade as we saw with the two jets from the cliff face next to the Hapi region. Without the highly effective insulation of the dust layer, 67P and all other comets, would all have evaporated away by now.
Similar (but likely less detailed) temporal variations have been noticed in other comets before. It does seem to suggest that gasses (mainly CO, CO2 and H2O) are not in a heterogeneous mixture, but rather “stratified” or “lumped” or otherwise able to sublimate independently (properties of clathrates.. ?)
Thinking back on Rosettas arrival at 67p: an (unexpected) “flare up” occured prior to first orbit; could it have been carbon monoxide sublimation?
Now a near-even distribution of water and CO2/CO is found – maybe later on this will move towards relatively more water?
The evolution of gases to the coma is going to be a complex subject. There are unknown gases involved with clathrates of unknown forms of ices.
Here, FWIW, is one of my working lists of “gas properties” that I am compiling (hope it makes it thru the formatting process):
===
water
triple point 0deg C 273K
sublimation of ice -23degC -10degF 250K
ammonia
Melting point −77.73 °C (−107.91 °F; 195.42 K)
Boiling point −33.34 °C (−28.01 °F; 239.81 K)
carbon dioxide
melting point −56.6 °C; −69.8 °F; 216.6 K (at 1 atu)
sublimation −78.5 °C; −109.2 °F; 194.7 K
carbon disulphide
carbon monoxide
Melting point −205.02 °C (−337.04 °F; 68.13 K)
Boiling point −191.5 °C (−312.7 °F; 81.6 K)
cyanogen
Melting point −28 °C (−18 °F; 245 K)
Boiling point −21.1 °C ( −6.1 °F; 252.0 K)
formaldehyde
hydrogen cyanide
Melting point −14 to −12 °C; 7 to 10 °F; 259 to 261 K
Boiling point 25.6 to 26.6 °C; 78.0 to 79.8 °F; 298.7 to 299.7 K
hydrogen sulphide
methane
Melting point −182.5 °C; −296.4 °F; 90.7 K
Boiling point−161.49 °C; −258.68 °F; 111.66 K
methanol
Melting point −97.6 °C (−143.7 °F; 175.6 K)
Boiling point 64.7 °C (148.5 °F; 337.8 K)
sulphur dioxide
ALL AT 1 ATU (101.325 kPa)
triple points
Temp @Press
Ammonia 195.40 K (−77.75 °C) 6.076 kPa (0.05997 atm)
Carbon dioxide 216.55 K (−56.60 °C) 517 kPa (5.10 atm)
Carbon monoxide 68.10 K (−205.05 °C) 15.37 kPa (0.1517 atm)
Methane 90.68 K (−182.47 °C) 11.7 kPa (0.115 atm)
Water 273.16 K (0.01 °C) 0.6117 kPa= 611.73 Pa (0.006037 atm)
===